AI Optics receives FDA 510(k) for retinal screening camera
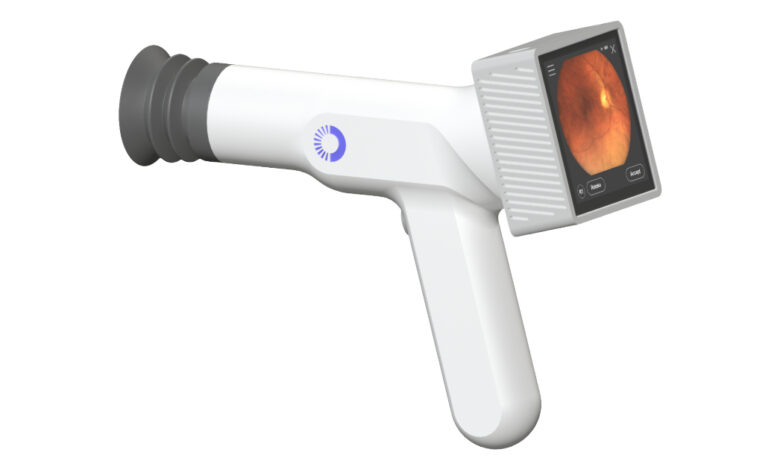
AI Optics, an AI-enabled medical device company, has received FDA 510(k) clearance for its Sentinel Camera.
The handheld retinal imaging system captures high-quality images of the human eye, helping to address gaps in retinal disease screening through a portable and accessible tool.
The aim of the Sentinel Camera is to enable point-of-care retinal imaging that would eliminate the need for some patients to visit an eye specialist’s office.
According to the company, the design of the camera was developed to meet the needs of general healthcare providers, including primary care, optometry, retail health clinics and home health settings, and ensure accessibility and practicality in these environments.
The Sentinel Camera supports DICOM-compliant image formats, which allows integration with Electronic Health Record (EHR) systems to streamline care coordination, billing and data sharing.
When combined with AI Optics’ upcoming AI capabilities, the system is intended to further enhance point-of-care screening workflows and diagnostic efficiency.
AI Optics is collaborating with NYU Langone Health to advance the accessibility and implementation of retinal screening technology.
“Our vision with the Sentinel Camera is to eliminate barriers to retinal screening and ensure that every patient who needs screening has access,” Luke Moretti, cofounder and CEO of AI Optics, said in a statement.
“This FDA clearance not only validates our significant progress to breaking down screening barriers but also sets the stage for our future AI-powered screening solutions, which will integrate seamlessly with the Sentinel Camera to deliver unparalleled accessibility and efficiency in retinal disease detection.”
THE LARGER TREND
In 2024, Google licensed its AI model focused on detecting diabetic retinopathy to partners in Thailand and India, two countries the tech giant says have a shortage of eye specialists.
Google Research teams partnered with foundational research partners Aravind Eye Hospital in India and Rajavithi Hospital in Thailand nearly a decade ago to research whether AI could be used to help reduce preventable blindness caused by diabetic retinopathy.
Retina Labs markets a cloud-based teleophthalmology platform that offers an integrated solution that combines advanced ocular imaging, clinical interpretation and reporting tools with electronic referrals. Retinal photos are taken using a retinal camera operated by a medical assistant, technician or nurse.
In 2024, Toku unveiled CLAiR which provides noninvasive evaluation for the risk of cardiovascular disease at the point of care using retinal images during a routine eye exam. The AI-powered CLAiR technology identifies elevated CV risk by analyzing minute changes in the retina and its vasculature. CLAiR results can be shared with a patient’s primary care physician.




